
Injection molding has advanced significantly with the use of biocompatible fluoropolymers. Fluoropolymers are a type of synthetic polymer that contains fluorine atoms, providing unique properties such as high thermal stability, chemical resistance, and low friction. When these fluoropolymers are made biocompatible, they become suitable for various medical and healthcare applications.
The use of biocompatible fluoropolymers in injection molding offers several advantages:
- Biocompatibility: These materials are designed to be compatible with living tissues and can be safely used in medical devices and implants. This makes them ideal for applications where direct contact with the human body is necessary.
- Chemical Resistance: Fluoropolymers are known for their excellent chemical resistance. In medical environments, where exposure to various chemicals is possible, these materials provide a protective barrier against corrosion and degradation.
- High Thermal Stability: Fluoropolymers can withstand high temperatures without losing their structural integrity. This property is crucial in medical applications where sterilization processes involve elevated temperatures.
- Low Friction: The low coefficient of friction of fluoropolymers makes them suitable for applications where smooth surfaces and minimal friction are essential, such as in medical devices that come into contact with bodily fluids.
- Precision Molding: Injection molding allows for the production of complex and precise shapes. This is particularly beneficial in medical device manufacturing where intricate designs are often required for optimal performance.
- Cost-Effectiveness: Injection molding is a cost-effective manufacturing process, especially for high-volume production. This makes it an attractive option for producing medical components and devices on a large scale.
Applications of biocompatible fluoropolymers in injection molding include the manufacturing of components for medical devices such as catheters, connectors, seals, and implantable devices.
It’s important to note that the adoption of these materials in medical applications requires compliance with regulatory standards and guidelines to ensure the safety and efficacy of the produced devices. Manufacturers must adhere to strict quality control measures to meet the stringent requirements of the healthcare industry.
Expertise in custom plastic injection applications not only ensures the technical accuracy of the manufactured parts but also builds trust and fosters long-term partnerships between manufacturers and clients.
For more information on the top of Biocompatible Fluoropolymer Advances, please see our white paper at:
https://performanceplastics.com/blog/biocompatible-fluoropolymers/
Performance Plastics is a world-class complex injection molding company. We work with our customers to leverage the capabilities of ultra and high-performance materials such as PEEK, Torlon®, Ultem®, PFA, FEP, PVDF, and ETFE and can achieve extreme dimension or tight tolerance injection molded parts.
For more information on Performance Plastics’ capabilities, please contact Rich Reed, Vice President of Sales & Marketing at [email protected], or visit our website at www.performanceplastics.com.

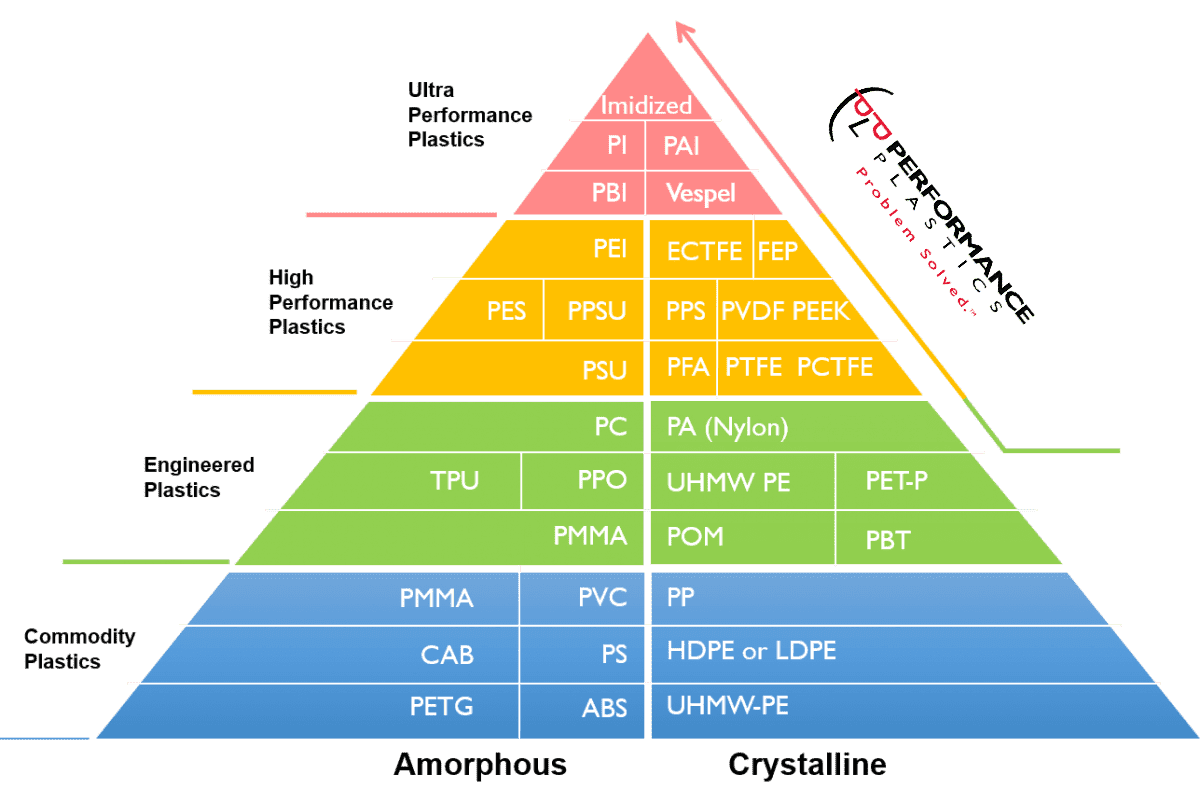 Plastics can be considered high-performance for a variety of reasons. The specific application and the performance criteria are the most important. Here are some characteristics that can contribute to a resin being classified as a high-performance plastic.
Plastics can be considered high-performance for a variety of reasons. The specific application and the performance criteria are the most important. Here are some characteristics that can contribute to a resin being classified as a high-performance plastic.












